Comets Are Oxygen Factories

When Europe’s Rosetta mission detected molecular oxygen venting into space from comet 67P/Churyumov-Gerasimenko in 2015, scientists were puzzled.
Molecular oxygen is composed of two oxygen atoms (O2). It’s the same stuff that we breathe here on Earth, but it’s highly unstable in space. O2 is highly reactive and attaches itself to other chemicals like hydrogen (creating water) and carbon (creating carbon dioxide) very quickly. Finding O2 fizzing from something as ancient as an icy comet just didn’t make any sense.
Interplanetary space is one huge particle accelerator. Charged particles zoom around unimpeded and, in the case of comets, they produce a LOT of particles.
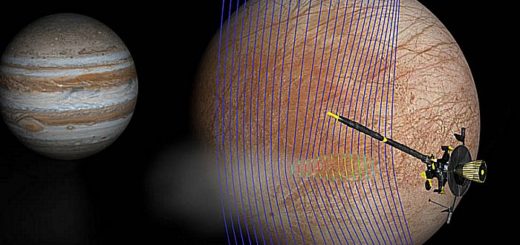
Comets arose from the primordial mess that built our solar system 4.6 billion years ago, and comet 67P is no different. When astronomers sent Rosetta to the ancient icy object, the spacecraft’s mission was to understand the comet’s composition so scientists could, ultimately, use it as a time capsule to see what the early solar system looked like.
So, finding O2 erupting from the comet could only mean one thing – this oxygen had been locked inside the comet’s ice for 4.6 billion years and, as it approached the sun, the oxygen was venting into space as the sunlight heated up the comet’s nucleus, liberating the ancient O2 from its icy mass.
But a chemical engineer who works at the California Institute of Technology (Caltech) thinks something else is going on and that the O2 Rosetta observed isn’t ancient at all.
He thinks comet 67P is, in fact, a molecular oxygen factory.
From Laboratory to Interplanetary Space
Konstantinos P. Giapis studies the chemical reactions between energetic ions colliding with semiconductor surfaces. By experimenting with these reactions, the chemical engineer can create more efficient microchips for the computers on our desks and the smartphones in our pockets. But when Giapis heard about Rosetta’s strange oxygen discovery – millions of miles away in deep space – he realized he might already know the answer in his lab.
“I started to take an interest in space and was looking for places where ions would be accelerated against surfaces,” says Giapis in a press release. “After looking at measurements made on Rosetta’s comet, in particular regarding the energies of the water molecules hitting the comet, it all clicked. What I’ve been studying for years is happening right here on this comet.”
Interplanetary space is one huge particle accelerator. Charged particles zoom around unimpeded and, in the case of comets, they produce a LOT of particles.
As any comet approaches the sun, solar heating will cause the ice particles contained within the nucleus to sublimate — when volatiles like water are converted straight from a solid (ice) to vapor, skipping the liquid phase. As water molecules are released from the comet and ejected into space, the sun’s ultraviolet light ionizes the molecules. When this happens, the molecules get swept up in the solar wind and blown back to the comet’s surface at high speed.
During laboratory tests, Giapis showed that when these water particles hit the comet’s surface, they attach themselves to the oxygen atoms contained in other molecules, such as iron oxide (rust) and silicon dioxide (sand). After the collision, new chemical bonds are made, and molecular oxygen is produced and vented into space.
Full Width
This illustration aims to explain how high-speed water molecules (left) interact with rust and sand on the surface of a comet to form a plume (right) that contains molecular oxygen. (Oxygen atoms are red, and hydrogen ones are blue.)
CALTECH
“We have shown experimentally that it is possible to form molecular oxygen dynamically on the surface of materials similar to those found on the comet,” says postdoctoral scholar Yunxi Yao in the press release. Yao also works at Caltech.
“We had no idea when we built our laboratory setups that they would end up applying to the astrophysics of comets,” adds Giapis. Giapis and Yao’s findings have been published in the journal Nature Communications.
Alien Biomarkers?
So, the original Rosetta observations likely weren’t of primordial molecular oxygen; this ancient comet appears to be producing new oxygen molecules from the water ices that are sublimating and colliding with other chemicals.
Though this discovery might solve Rosetta’s oxygen mystery, it poses a different challenge for another area of astronomy.
When looking for extraterrestrial life elsewhere in the galaxy, astrobiologists hope to eventually have powerful telescopes and high-resolution spectrometers that can be used to probe the atmospheres of exoplanets light-years distant. Thus equipped, they’ll look for chemicals that are known to be associated with life (as we know it).
These chemicals are known as “biomarkers,” and the discovery of O2 in one of these distant atmospheres may lead us to believe that something biological is producing the molecule, as plants do on Earth through photosynthesis. But now we know that O2 can be produced in space “abiotically” (not through biological processes), and other star systems may produce O2 similarly, potentially creating a “false positive” in our search for extraterrestrial biomarkers.


 Creators of mankind
Creators of mankind Description of “Tall white aliens”
Description of “Tall white aliens” Where they came from?
Where they came from? About hostile civilizations
About hostile civilizations The war for the Earth
The war for the Earth “Tall white aliens” about eternal life
“Tall white aliens” about eternal life Video: “Nordic aliens”
Video: “Nordic aliens” Aliens
Aliens Alien encounters
Alien encounters The aliens base
The aliens base UFO
UFO Technology UFO
Technology UFO Underground civilization
Underground civilization Ancient alien artifacts
Ancient alien artifacts Military and UFO
Military and UFO Mysteries and hypotheses
Mysteries and hypotheses Scientific facts
Scientific facts